Chemistry in the Atmospheres and Circumstellar Envelopes of Cool Stars
The origin of the gas and grains that accumulated to form the solar nebula and the chemical reprocessing of these materials during the formation of the Earth and other bodies in the solar system are fundamental questions. We recently completed models of grain condensation in cool, carbon-rich stars. The comparison of our calculated trace element abundance patterns with observed patterns shows that the presolar silicon carbide (SiC) grains found in meteorites came from at least three different types of carbon stars, which our modeling identifies. Our models identify some of the stellar sources that contributed dust to the solar nebula. We are modeling the chemical composition of other presolar grains, namely the titanium carbide (TiC) grains, also found in meteorites. This work will help constrain the origin and formation conditions of these presolar grains. We are also modeling the gas chemistry of cool, carbon-stars. This work is important for interpreting existing astronomical observations of carbon stars and for guiding the direction of future observations.
Atmospheric Chemistry of Brown Dwarfs
Through the use of thermochemical equilibrium calculations, we have modeled the elemental abundances of the brown dwarf, Gliese 229B. These calculations provide a foundation for interpreting the existing spectroscopic observations and for guiding future spectroscopic studies of Gliese 229B. These calculations include predictions that refractory elements are removed by condensate cloud formation at temperatures above 1600 K, and predictions of the major C, N, S, P, Cl, and F gases in the atmosphere. Furthermore, rapid vertical mixing, much like that of the Jovian planets of our solar system, may increase the abundance of some species to a greater proportion than that which thermochemical equilibrium would suggest.
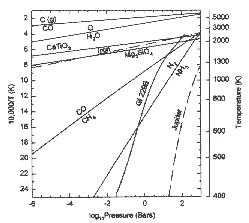
A comparison of the pressure (P)-temperature (T) profiles for the atmospheres of Gliese 229B (Teff=960 K and g=1000 ms-2 from Marley et al. 1996) and Jupiter with some important thermochemical phase boundaries in a solar composition gas. As explained in the text, the lines labeled C/CO, CO/CH4, O/H2O, and N2/NH3 are boundaries along which carbon, oxygen, and nitrogen gases have equal abundances. The CaTiO3 (perovskite) condensation line is representative of the pressure-dependent temperatures at which other refractory elements such as Al, Zr, V, and the lanthanides condense from solar gas. The abundant rock-forming elements (Mg, Si, Fe) condense as iron and Mg2SiO4 (forsterite).
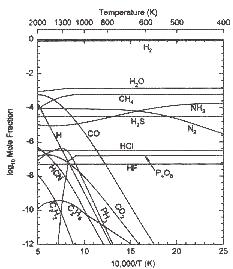
Thermochemical equilibrium abundances of the major H, O, C, N, S, P, Cl, and F gases along with the adopted P-T profile for the atmosphere of Gliese 229B. The mole fraction of the different gases are equal to the partial pressure of a gas divided by the total pressure. These calculations assume solar elemental abundances (Anders & Gravesse 1989).
Both graphs courtesy of The Astrophysics Journal, 472: L39, 1996 Nov. 20
Click here to view the paper “Atmospheric Chemistry of the Brown Dwarf Gliese 229B: Thermochemical Equilibrium Predictions.”
Atmospheric Chemistry of Jovian Planets
We have been actively involved in this area for almost 20 years and see our research efforts continuing into the future. We have developed comprehensive models of the atmospheric chemistry of the gas planets Jupiter and Saturn. These models provide a foundation for interpreting existing spectroscopic observations, for guiding future observations from Earth-based and spacecraft platforms, and for designing experiments on future spacecraft missions. In particular, our models show that some gases observed in the upper atmosphere of Jupiter and Saturn (e.g., carbon monoxide CO, phosphine PH3, arsine AsH3, germane GeH4) originate from regions about 1000 kilometers below the visible cloud tops.
Oxygen Isotope Model for Chemical Composition and Physical of Terrestrial Planets, Satelittes, and Asteroids
Dr. Lodders developed an oxygen isotope mixing (OIM) model for the chemical composition and physical properties of rocky planets and asteroids in her doctoral thesis in 1991. The basis of the OIM model is that the mean oxygen isotopic composition of rocky planets, satellites, and asteroids is determined by the oxygen isotopic composition of the mixture of nebular material accreted to form these bodies. Oxygen is generally the most abundant element in rock and depending on the existence and size of an iron-bearing core is either the first or second most abundant element in a rocky planet, satellite, or asteroid. Thus the OIM model is unique because planetary compositions and properties are determined using the first (or second) most abundant element in the planet, instead of using trace elements as done in many other models. Chondritic meteorites are the best examples of nebular material that we have and these are used for the modeling.
Dr. Lodders and Professor Fegley used the OIM model to predict many chemical and physical properties of Mars. This was done using the widely held assumption that Mars is the parent body for the SNC (Shergottite – Nakhlite – Chassignite) meteorites. The oxygen isotopic compositions of the different SNC meteorites are known and their average can be calculated. Then, the average oxygen isotopic composition of the SNC meteorites gives the average oxygen isotopic composition of Mars. The bulk chemical composition of Mars follows from mass balance calculations which mix together different amounts of chondritic material to match the average oxygen isotopic composition of Mars. The size of the Martian core and of the silicate fraction of Mars (that is the mantle + crust), the composition of the core and mantle + crust, the density of the planet, the moment of inertia of the planet and other properties can all be calculated. The figures below give some of the background and results of the OIM model for Mars. Further details can be found in the paper “An Oxygen Isotope Model for the Composition of Mars” published by us in Icarus in April 1997 (vol. 126, pp. 373-394). Read this paper by clicking here.
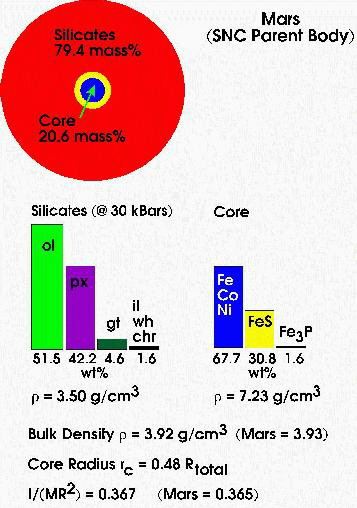
Figure 1. A cartoon summarizing some of the major predictions of the OIM model. The OIM model predicts a bulk density and moment of inertia factor very similar to the observed values for Mars. The predicted core size and composition and size and composition of the Martian mantle + crust are also shown along with other properties. Based on results given by Lodders and Fegley (1997).
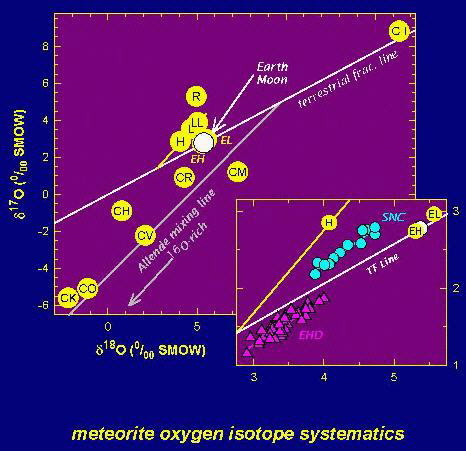
Figure 2. A plot showing the oxygen isotope compositions of the Earth, Moon, Mars, the asteroid 4 Vesta, and of different types of meteorites. Mars is generally believed to be the source of the SNC meteorites. Vesta is generally believed to be the source of the eucrite, howardite, and diogenite (EHD) meteorites. This figure is a modification of Figure 1 in Lodders and Fegley (1997). The data are from papers published by R.N. Clayton and colleagues, which are cited in our paper.
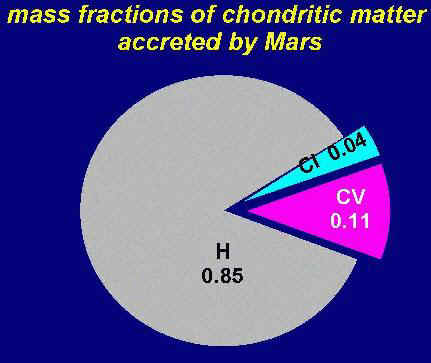
Figure 3. A pie diagram showing the mass fractions of chondritic matter accreted by Mars. Based on results given by Lodders and Fegley (1997).